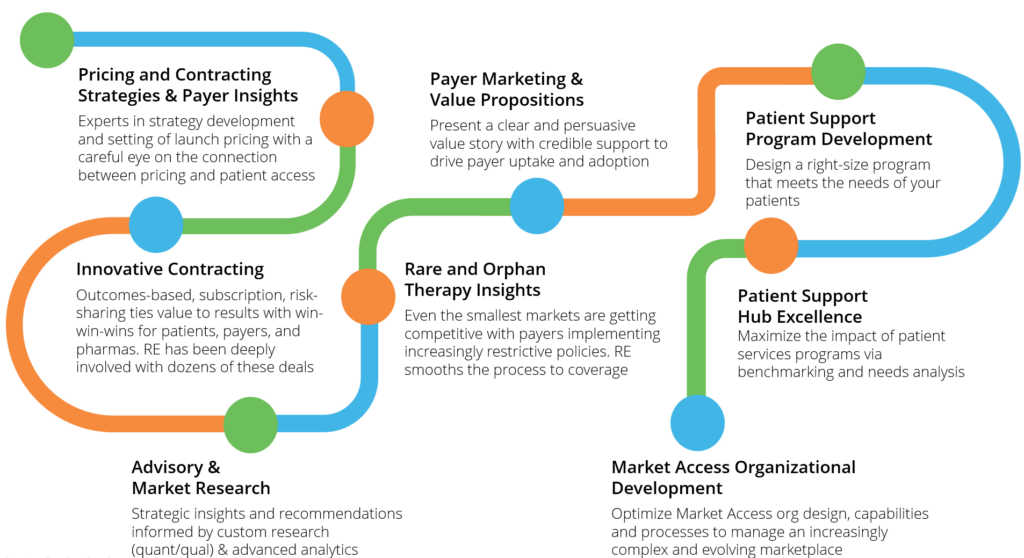
Solutions
Real Endpoints brings a wide range of strategic and operational market access solutions to our clients.
Platforms
Real Endpoints offers platforms to help our clients maximize the potential of their market access teams.
Solutions
News and Events

Real Endpoints announces four biopharma clients have signed on as customers for RE Assist, a tech-enabled AI platform providing real-time foundation fund information to Medicare patients
by Real Endpoints
Real Endpoints, the leading market-access platform and advisory firm, announces that four biopharma companies with combined 2022 sales of nearly $8 billion have signed contracts to offer Real Endpoint's RE Assist tool to support Medicare patients in need of deductible and copay assistance. RE Assist provides actionable, comprehensive foundation fund information in a compliant and easy to navigate format.
April 13, 2023
Read Here >
Real Endpoints Marketplace announces collaboration with bluebird bio to help scale delivery of a first-of-its-kind value-based contract for one-time gene therapy
by Real Endpoints
The risk-sharing agreement removes economic uncertainties, enabling regional and mid-sized health plans to provide patients with transfusion-dependent beta-thalassemia access to ZYNTEGLO
October 4, 2022
Read Here >
Gene Therapies: Building sustainable commercial strategies
Gene therapy companies are part of a scientific vanguard — but must blaze new commercial pathways as well. Real Endpoints is bringing together a select group of senior gene therapy executives to discuss, in an informal resort setting and under Chatham House rules, future best practices in the commercialization of this utterly novel class of therapies. In case studies, group sessions, and one-on-one conversations, this meeting will delve into issues such as the challenges of launching one-time cures in markets dominated by chronic therapies; making the cost-effectiveness case in a world of short-term insurance models; and the latest in innovative contracting approaches.
May 17, 2023
-
May 18, 2023
